Today we tried our fuel that we made. We went outside and saw a bus. There were workmen who poured the fuel into the bus. When the workmen were done pouring the fuel into the car, they started the car. (drum roll) They ignited the bus and the engine ran!! Yay we were so happy that they bus ran. We were so happy, but a second later our principle came out and told us that we were going to have a lockdown. Everybody ran inside and went to the classroom. We all had to sit down and wait for further instructions. It took about 20 minutes untill the lockdown was finally over. After the lockdown was over we all waited for about 10 minutes and we got to eat ICE CREAM!! YAY! So this is basically how the day went. Mr. Ham (somebody invited by Ms. Rioux, maybe the head of the bus department) e-mail Ms.Rioux saying that the fuel that we made worked and it smelt like french fries.
Thank You, Ms. Rioux I will always remmeber this!
Tuesday, May 24, 2011
Wednesday, May 18, 2011
Experimenting During Class #7
Today in class we all went to go squeeze the debris out of our bottles. First we all went to go get all of our bottles. Then some of us had to wait in line because we only had two buckets this time. So some of us waiteed in line while the other people were squeezing thier debris out. When you squeeze the debris out, one person has to hold your bottle while the other one unscrews the cap. You slowly unscrew the cap and the other person slowly squeezes out the debris. After my bottle was clean out of debris I poured the oil into the beakers. Lastly once the beakers were done I had to clean my bottle with Dawn soap. We also were assigned to find out a way to test it, like a physical property test. I did some reasearch and found out that we can find the boiling point, the density, or the pH of the oil.
Tuesday, May 17, 2011
Experimenting During Class #6
Today in class we made another batch of biodiesel. This time everything went more smoother than last time. When we started the experiment everybody just went to do a job that was open. For instance there was about four people putting the oil into the bottles, and there were two people measuring the sodium hydroxide. The small glass bottles that we used last time to put the methanol and sodium hydroxide in got dirty and people had to clean them out, so we had about three people cleaning them out. The people that got done first were the people who were putting in the oil in the bottles. Our teacher went ahead a took the small glass bottles that were already dry and started to pour the methanol in them. The people with the sodium hydroxide weren't done measuring and took the longest out of all the three stations. After the sodium hydroxide people were done measuring everyone pour the sodium hydroxide in with the methanol and shook the bottle until all of the sodium hydroxide was gone. Lastly when waited in line to get our methanol,sodium hydroxide and our oil to get mixed together. Our teacher shook it 8 times this time. We can also try to test the oil by using a physical property test by it's boiling point or how dense it is.
Sunday, May 15, 2011
Experiment During Class #4 (May 12)
Today I am going to type what happened in class three days ago. First our teacher showed us a demonstration on how to open our bottles. We first have to put the bottle over a bucket. Then you open the bottle you have to have one person untwisting it and one holding the bottle. The person unscrewing the bottle unscrews it slowly and the person holding it will slowly squeeze the bottle to get the all the unused debris out. A lot of the oil in the bottle didn't settle down enough and even though when we squeezed out the unused debris we still had to let the oil settle for another day. How we got all of the unused debris was when we did our last experiment (read Experiment During Class #3) the methanol and the sodium hydroxide reacted with the oil and the oil produced all of the unneeded debris and things inside the bottle.
Wednesday, May 11, 2011
Experimenting During Class #3
Today we made biodiesel. We split into three teams. One team was the oil team, one was sodium hydroxide, and the last one was the methanol team. I was put in the sodium hydroxide, but I still saw what happened with the other teams. For the oil team what they had to do was pour 1L of used cooking oil into the 2L bottles. For the sodium hydroxide team, what we had to do was take 12 grams of sodium hydroxide and put them on petri dishes. Last but not least the methanol team had to pour 250mL of methanol into little jars. Everybody had to do 14 of everything because we had 14 people in our class.
After all 14 of the oil, sodium hydroxide, and the methanol were all done we had to go to the center of the table. Then everybody got a jar of methanol and one dish of sodium hydroxide. First we watched our teacher demonstrate how to put the sodium hydroxide into the methanol and shake it. Then we did it we had to shaked it too, and it took a long time for the sodium hydroxide to dissolve. After about 20 minutes or so the sodium hydroxide started to dissolve for some people and they went on to the next step. The next step is to go to the teacher and ask her to pour the liquid inside your 2L bottle. Lastly you tilt it up and down 4 times and put it upside down on top of a beaker. This is what happened in class today.
After all 14 of the oil, sodium hydroxide, and the methanol were all done we had to go to the center of the table. Then everybody got a jar of methanol and one dish of sodium hydroxide. First we watched our teacher demonstrate how to put the sodium hydroxide into the methanol and shake it. Then we did it we had to shaked it too, and it took a long time for the sodium hydroxide to dissolve. After about 20 minutes or so the sodium hydroxide started to dissolve for some people and they went on to the next step. The next step is to go to the teacher and ask her to pour the liquid inside your 2L bottle. Lastly you tilt it up and down 4 times and put it upside down on top of a beaker. This is what happened in class today.
Sunday, May 8, 2011
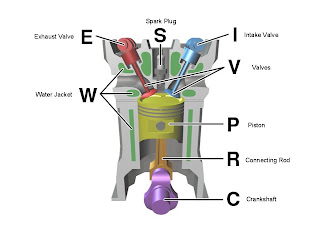
Diesel Engine
This is a picture of a diesel engine and its parts. The diesel engine works by these steps.
Step 1: When you turn the key to a diesel engine powered car the fuel is injected into the cylinders which cause massive heat. Depending on the temperature you might have to wait a while for the car to warm up.
Step 2: Once your done a light will appear in your car that says "Start". When you see it step on the accelerator and turn the key to start.
Step 3: Fuel pumps deliver the fuel from the fuel tank to the engine and goes through fuel filters.
Step 4: The fuel is pumped and pressurized into a delivery tube.
Step 5: The air that comes through an air filter meet in the cylinders with the fuel.
Step 6: Then you drive the car!
Step 1: When you turn the key to a diesel engine powered car the fuel is injected into the cylinders which cause massive heat. Depending on the temperature you might have to wait a while for the car to warm up.
Step 2: Once your done a light will appear in your car that says "Start". When you see it step on the accelerator and turn the key to start.
Step 3: Fuel pumps deliver the fuel from the fuel tank to the engine and goes through fuel filters.
Step 4: The fuel is pumped and pressurized into a delivery tube.
Step 5: The air that comes through an air filter meet in the cylinders with the fuel.
Step 6: Then you drive the car!
Thursday, May 5, 2011
Experimenting During Class #2
Today was the second day experimenting in class and the process went faster than I thought. The first thing that we did was put cheese cloth over 2 buckets. While we were putting the cheese cloth over the buckets, other people went to go pump the oil from a barrel into other buckets. After the other people were done pumping the oil into the buckets we poured the oil into the filter. It took us a while to pour the oil into the buckets, but we got faster at pouring the oil. After we were done pouring the oil through the filter our teacher told us that we had to filter it again for a total of two times. So she told us that there was a hose outside to wash the barrels off. I went outside to help wash the barrels off. There was people inside and outside of the classroom, so we did a cycle. We couldn't boil the oil today because of what happened yesterday, but there was some people who will stay after school on next Monday to heat up the oil. I hope that tomorrow that the filtering people won't make a big mess like today.
Wednesday, May 4, 2011
Experimenting During Class
Today in class we filtered oil. It took us about 15 minutes to write our procedures down and talk about it amongst ourselves. After we were done discussing about how to filter the oil we then went on to the experiment. First, we took cheese cloth and put it over a big funnel. Second, we put the funnel over beaker. Third, we pour the oil into the funnel, but we made sure that the funnel and beaker were over the bucket of oil because we didn't want the oil to get on the table. Fourth, after we had pour the oil into the beakers we put them on hot plates. Here comes the trouble..... We put about 10 beakers on the hot plates and it had to boil up to 100 degrees Celsius. I was one of the people that had to check the temperature, and I waited for about 10 minutes wondering "why isn't the oil boiling?" I told the people in my class that the temperature wouldn't go up, but they just told me to keep waiting. So I waited for about 5 minutes this time and the temperature still wouldn't go up so I think somebody told our teacher that the hot plates didn't work. Our teacher came by, checked the hot plates and power strips then realized that the circuit overloaded. So we basically didn't get anything done today. Even though we didn't get anything done today we still have to filter the oil by Friday, so we have to find another, faster way to filter the oil. If there's one thing I've learn today it's that you should always check to make sure everything works!
Tuesday, May 3, 2011
Hypothesis and Materials
If we clean the oil then the oil will get cleaner than it was originally because we will take out some dirt in the oil.
Materials: coffee filter, cheese cloth, hotplate, big funnels, cooking thermometers and buckets.
The steps to clean the oil is listed below
Step 1: Heat the oil on a hot plate until it reaches 35 degrees C.
Step 2: Then pour the oil through one of the filters that we have. (coffee filter or cheese cloth)
Step 3: After that, heat the oil again until it reaches 100 degrees C and when the boiling starts to slow down turn it up to 130 degrees C.
Step 4: Then let the oil sit for about 10-15 minutes.
Step 5: Lastly you have your oil!
Materials: coffee filter, cheese cloth, hotplate, big funnels, cooking thermometers and buckets.
The steps to clean the oil is listed below
Step 1: Heat the oil on a hot plate until it reaches 35 degrees C.
Step 2: Then pour the oil through one of the filters that we have. (coffee filter or cheese cloth)
Step 3: After that, heat the oil again until it reaches 100 degrees C and when the boiling starts to slow down turn it up to 130 degrees C.
Step 4: Then let the oil sit for about 10-15 minutes.
Step 5: Lastly you have your oil!
Monday, May 2, 2011
Research and Oil
From the research that I've done about biodiesel I find that it's really hard to make biodiesel with used oil.
Fortunately I've found one resource that I think is really good and the the website is called http://www.journeytoforever.org/biodiesel_mike.html . The website tells you how to make biodiesel with used oil and safety tips. I've learned that when your using used oil to make biodiesel it's harder to make than with new or fresh oil because when it's used oil there are more pieces in the oil that you have to take out. When you use new oil it's a bit easier because when it's new there isn't anything inside so you can experiment faster, but when you use used oil it's harder because you have to filter the oil first then you can experiment.
How you filter used oil is a very simple task. What you can do is you can take three 2 liter bottles and stack them up like this picture https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEgszg_TPqeGeHMSFUwyqK9pPSqPg_yV4ax1IYgG87h7dsUvfjRlmSBk6g1CcJc3lrcDW2r9KAbHvAP_dsm-yKcx2CrqSp8EbG0wio4fcEIUbRD2B0P_HJ_4_qlb6DbVViPnmjyyoX2klfK9/s1600/Bio+Fuel+025.JPG . When you stack them up make sure to put the tights in the middle bottle first then the T-shirt in the first bottle. When your done stacking the bottles on top of each other and have the tights and T-shirt in the bottles then you can pour the oil in from the top and let it filter. Once your done pouring the oil TA-DA your done! You've filter oil!
Fortunately I've found one resource that I think is really good and the the website is called http://www.journeytoforever.org/biodiesel_mike.html . The website tells you how to make biodiesel with used oil and safety tips. I've learned that when your using used oil to make biodiesel it's harder to make than with new or fresh oil because when it's used oil there are more pieces in the oil that you have to take out. When you use new oil it's a bit easier because when it's new there isn't anything inside so you can experiment faster, but when you use used oil it's harder because you have to filter the oil first then you can experiment.
How you filter used oil is a very simple task. What you can do is you can take three 2 liter bottles and stack them up like this picture https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEgszg_TPqeGeHMSFUwyqK9pPSqPg_yV4ax1IYgG87h7dsUvfjRlmSBk6g1CcJc3lrcDW2r9KAbHvAP_dsm-yKcx2CrqSp8EbG0wio4fcEIUbRD2B0P_HJ_4_qlb6DbVViPnmjyyoX2klfK9/s1600/Bio+Fuel+025.JPG . When you stack them up make sure to put the tights in the middle bottle first then the T-shirt in the first bottle. When your done stacking the bottles on top of each other and have the tights and T-shirt in the bottles then you can pour the oil in from the top and let it filter. Once your done pouring the oil TA-DA your done! You've filter oil!
Subscribe to:
Comments (Atom)